NETWORKING IS KEY! –
Studies and Statistics for Integrated Research and Projects and More
Background
The clinical trial unit within the Children’s Cancer Research Institute (CCRI), the Ruth Ladenstein Group is focused on enabling better outcomes for children and adolescents across all Paediatric Cancer entities. A key role and involvement in many European Paediatric Cancer Networks foster innovation to the field and allow us to tackle inequalities across Europe.
Our Research
Networking is key
Ruth Ladenstein Goup’s work is based on close collaboration with international clinical trial groups and research communities. Together they aim at a better understanding of disease profiles, development of stratifying diagnostic markers facilitation of optimized modern diagnostics and risk-adapted treatments with traditional chemotherapy as well as immunotherapy alongside with respective other treatment modalities including surgery, stem cell transplantation, targeted therapies. For more details refer to the S²IRP section.
Key collaboration platforms and projects
AGPHO /Austrian Paediatric Society (ÖGKJ)
Ruth Ladenstein coordinates the Austrian Working Group for Paediatric Haematology-Oncoloy (AGPHO) since 2006, in particular the group’s collaborative activities across Austrian centres including meetings to share recent and updated results of past and active international trials and registries to provide up to date knowledge in the field which in turn is highly relevant for daily clinical practice.
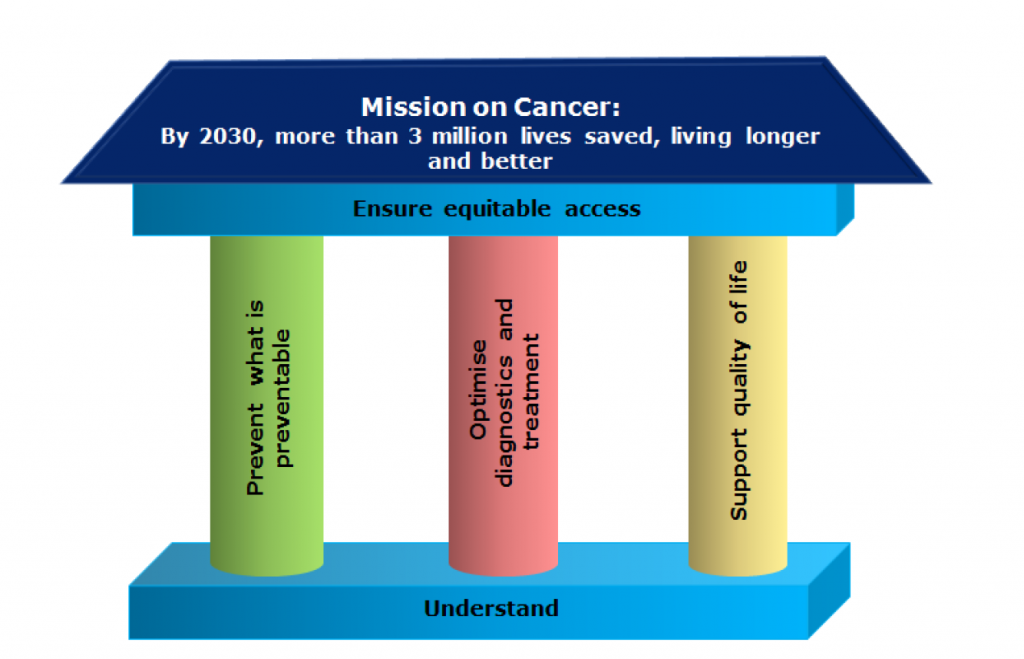
CANCER MISSION
EU Missions are a new way to bring concrete solutions to some of their greatest challenges. They have ambitious goals and will deliver tangible results by 2030. They will deliver impact by putting research and innovation into a new role, combined with new forms of governance and collaboration, as well as by engaging citizens. EU Missions are a novelty of the Horizon Europe research and innovation programme for the years 2021-2027.
The 4 Mission objectives
- Understanding of cancer
- Prevention and early detection
- Diagnosis and treatment
- Quality of life for patients and their families
Prof. Ladenstein was a member of the first Cancer Mission Bord contributing to the outline of the European Commission’s Cancer Mission programme.
ERN PAEDCAN
The European Reference Network on Paediatric Cancer (ERN PaedCan) was established in 2017 as an EU funded initiative to reduce inequalities in childhood cancer survival by providing high-quality, accessible and cost-effective cross-border healthcare to children and adolescents with cancer, regardless where they live. To make specialised know-how and life-saving paediatric oncology treatments broadly accessible, the ERN PaedCan created a roadmap of healthcare centres that are worldwide renowned for their expertise in treating paediatric malignancies. This network facilitates the lives of both healthcare providers and of patients whose conditions require specialist expertise and tools not widely available due to low case volumes and a lack of resources. The PaedCan ERN also implements eHealth technologies and improves interoperability across different institutions (e.g. via a virtual paediatric oncology tumour board network to share expertise and advice). Finally, the ERN helps young patients and their families to make informed choices by providing clear information regarding access, quality, safety and reimbursement for treatments received in another EU country. All this aims to offer more treatment options and less red tape for young patients and their families no matter where they live.
OKIDS
The Medicines for Children Research Organization (OKIDS) was founded as a private-public partnership in 2012 and coordinates numerous tasks by networking the five Austrian university sites and the St. Anna Children’s Hospital through a national headquarter. The overarching aim is to increase availability of appropriate medicines for young patient groups. OKIDS provides study nurse capacity to its respective sites to foster industry-led and academic drug development for children and works closely with the sites’ leadership. OKIDS responds to feasibility requests and supports set up and management of clinical trials. Since its initiation, it has run 149 feasibilities for CROs, Pharma Industry, Enpr-EMA und the c4c IMI2 consortium, supported 376 studies and registries with an accrual of over 5800 patients at young age.
SIOPEN
The Society of Paediatric Oncology Europe Neuroblastoma Group (SIOPEN) fosters neuroblastoma research and enables long-term sustainability of the group. During her SIOPEN presidency Prof. Ladenstein has founded the SIOPEN Association with its siege in Vienna and is to date still supported by S²IRP working force. Currently, the SIOPEN association has 432 active members in 34 countries. A total of 29 countries are involved in SIOPEN studies.
SURVIVORSHIP PASSPORT
Austria is the first European country to include the Survivorship Passport for use by young people beating cancer in its Cancer Plan since 2013. The Survivorship Passport is a tool to provide all European childhood cancer survivors with optimal long-term care. It provides instant access to the medical history of patients who ended a cancer therapy, making survivors and healthcare professionals aware of the potential risks or late effects stemming from the previous disease and treatment received. With the support of the Austrian Ministry of Health and EU Projects the Survivorship passport will see its integration into Austrians E-health structure ELGA in 2023.
SIOP EUROPE
The European Society for Pediatric Oncology (SIOP Europe or SIOPE) is a pan-European organization representing all professionals working in the field of childhood cancers. From 2010 to 2012, Ruth Ladenstein has been elected for SIOPE president. As member of the SIOPE board since then, she and her colleagues oversee the work of the association. With more than 2000 members across 35 European countries, SIOPE is leading the way to ensure the best possible care and outcomes for all children and adolescents with cancer in Europe.
Projects and funding

-
Integrated and standardized NGS workflows for Personalised therapy (INSTAND-NGS4P)P
Principal Investigators: Ruth Ladenstein and Kaan Boztug
Coordinator: Kurt Zatloukal (Medical University Graz)
H2020 – Innovation Procurement, Grant Agreement ID – 874719
Duration: 01/01/2020 to 31/05/2025 -
Twinning research and education to improve survival in childhood solid tumors in Lithuania (TREL)
CCRI responsible Principal Investigator: Ruth Ladenstein
Coordinator: Jelena Rascon, Vilnius University Hospital Santaros Klinikos, Lithuania
H2020 – Twinning of research institutions, Grant Agreement ID – 952438
Duration: 01/01/2021 to 31/12/2023 -
Implementing the digital survivorship passport to improve person-centered survivorship care (PanCareSurPass)
CCRI responsible Principal Investigator: Ruth Ladenstein
Coordinator: Desiree Grabow, Universitätsmedizin Mainz
H2020 Grant Agreement ID – 899999
Duration: 01/03/2021 to 28/02/2025 -
European rare disease research coordination and support action (ERICA)
CCRI responsible Principal Investigator: Ruth Ladenstein
Coordinator: Alberto Pereira, Leiden University Medical Center, the Netherlands
H2020 Grant Agreement ID –964908
Duration: 01/03/2021 to 28/02/2025 -
European Joint Programme on Rare Diseases (EJP RD)
CCRI responsible Principal Investigator: Ruth Ladenstein
Coordinator: Daria Julkowska, Inserm, France
H2020 Grant Agreement ID – 825575
Duration: 01/01/2019 – 31/12/2023
Selected Articles
About Ruth Ladenstein
Prof. Ruth Ladenstein obtained her medical degree at the University of Vienna in 1982 and her Specialty Diploma of Paediatrics in 1992 after 3 years of Post Doc activities in Lyon and Paris /France where she also obtained a Diplôme in Oncology – ‘DISC’ (diplôme interuniversitaire de spécialité complementaire de cancerologie), University Claude Bernard, Lyon /Franc and completed a course for applied statistical methods and clinical research (CESAM) at the University Marie Curie, Paris /France. In 1998 she became Assoc. Professor of Paediatrics, University of Vienna and obtained in 2012 her Professor Degree from Ministry for Science and Research, Vienna. In 2007 she successfully certified as Senior Project Manager (IPMA-Level B®; Project Management Austria) and obtained a MBA at the University of Salzburg (2007-09) at the University of Salzburg in “International Health Care Management” (MBA). Up to today she is acting as Senior Consultant for Paediatric Oncology at the St. Anna Children’s Hospital
In 1996 she became head of the Clinical Trials Unit ‘Studies and Statistics for Integrated Research and Projects’ (S2IRP) at St. Anna CCRI and is chair of the Austrian Working Group for Paediatric Haematology-Oncology since 2006. She is board member of the SIOP Neuroblastoma Group (SIOPEN) since 1996, chaired the Society as President 2007-2013 and co-founded the SIOPEN association in 2009. She was President of SIOP EUROPE (2009 –2012) and is SIOPE board member since 2006 till today. Prof. Ladenstein is director of the Austrian Research Network for Paediatric Medicines (OKIDS) since 2012. Since 2017 she coordinates the European Reference Network for Paediatric Oncology (ERN PaedCan). She is a member of the Oncology Advisory Board of the Austrian Ministry of Health and was a member of the EU Mission Board for Cancer (Horizon Europe) from 2019 – 2021 and is since 2022 part of the Austrian Cancer Mission Action Group.
She received over 10 Awards in the field and is author of more than 200 peer reviewed papers and over 20 books chapters.
Studies & Statisics Integrated Research & Projects (S2IRP)
Find more information about the Clinical Trials Unit S2IRP here
Ruth Ladenstein, Head of S2IRP Email: ruth.ladenstein@ccri.at
Anke Emberger, Senior Operations Manager Email: anke.emberger@ccri.at
Ulrike Pötschger, Team Leader Statistics Email: ulrike.poetschger@ccri.at
